See Tolerability section and Summary of Product Characteristics (SmPC)1 for special warnings and precautions for use, interactions, and other safety-related information.

This is a promotional website with content intended for UK healthcare professionals only
Adverse events should be reported. Healthcare professionals are asked to report any suspected adverse reactions. UK reporting forms and information can be found via the Yellow Card Scheme www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Stores. Adverse events should also be reported to Neuraxpharm UK Ltd by emailing pv-uk@neuraxpharm.com
How to administer BUCCOLAM®?
• Hypersensitivity to the active substance, benzodiazepine or to any of the excipients. (sodium chloride, water for injections, hydrochloric acid and sodium hydroxide)
• Myasthenia gravis.
• Severe respiratory insufficiency.
• Sleep apnea syndrome.
• Severe hepatic impairment.
For infants between 3-6 months of age treatment should be in a hospital setting
Advise patients and carers to read the Patient Information Leaflet2
See Tolerability section and Summary of Product Characteristics (SmPC)1 for special warnings and precautions for use, interactions, and other safety-related information.
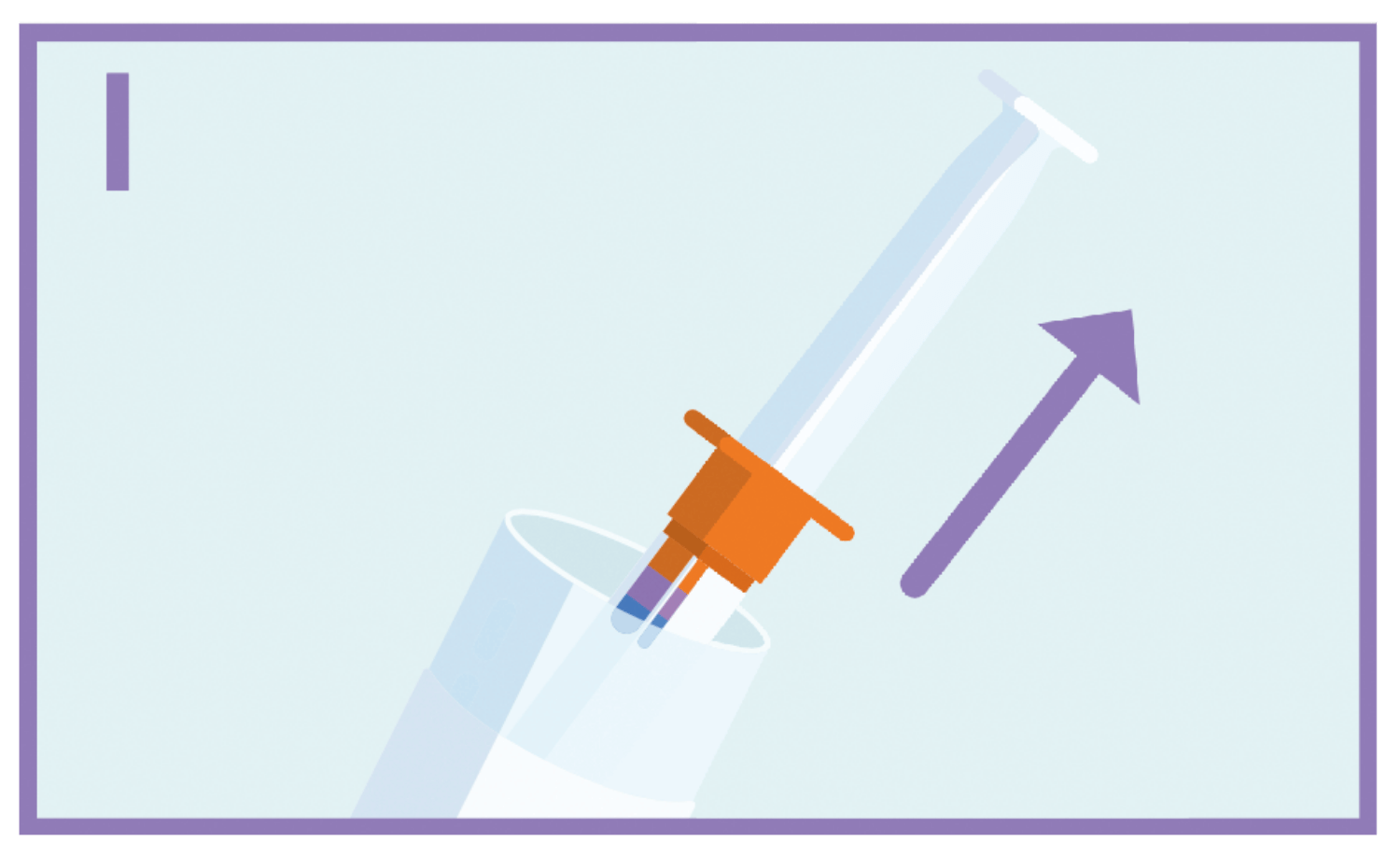
Hold the plastic tube and pull off the cap. Take the syringe out of the tube.
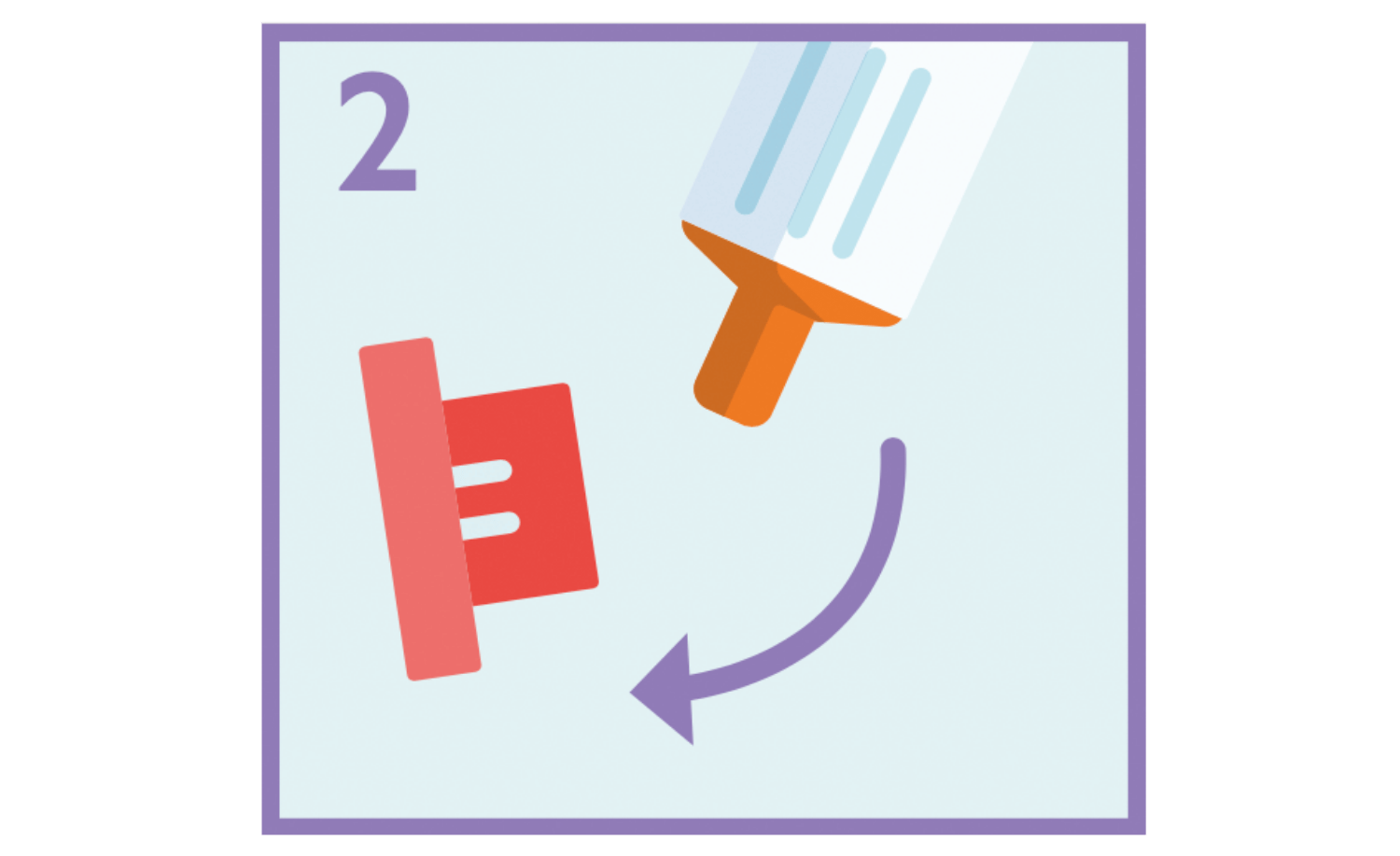
Pull the red cap off the tip of the syringe and dispose of it safely.
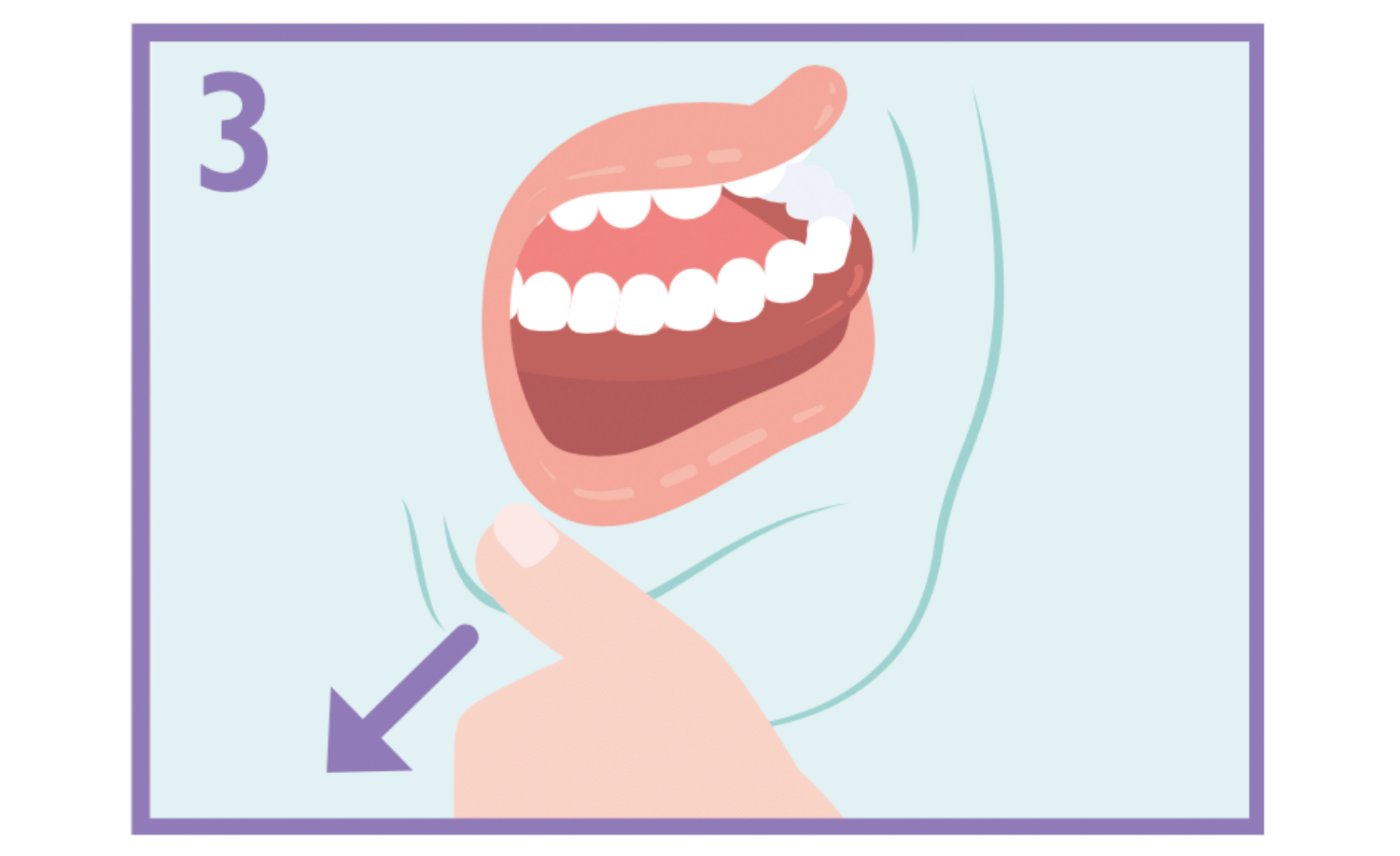
Using the finger and thumb gently pinch and pull back the patient’s cheek.
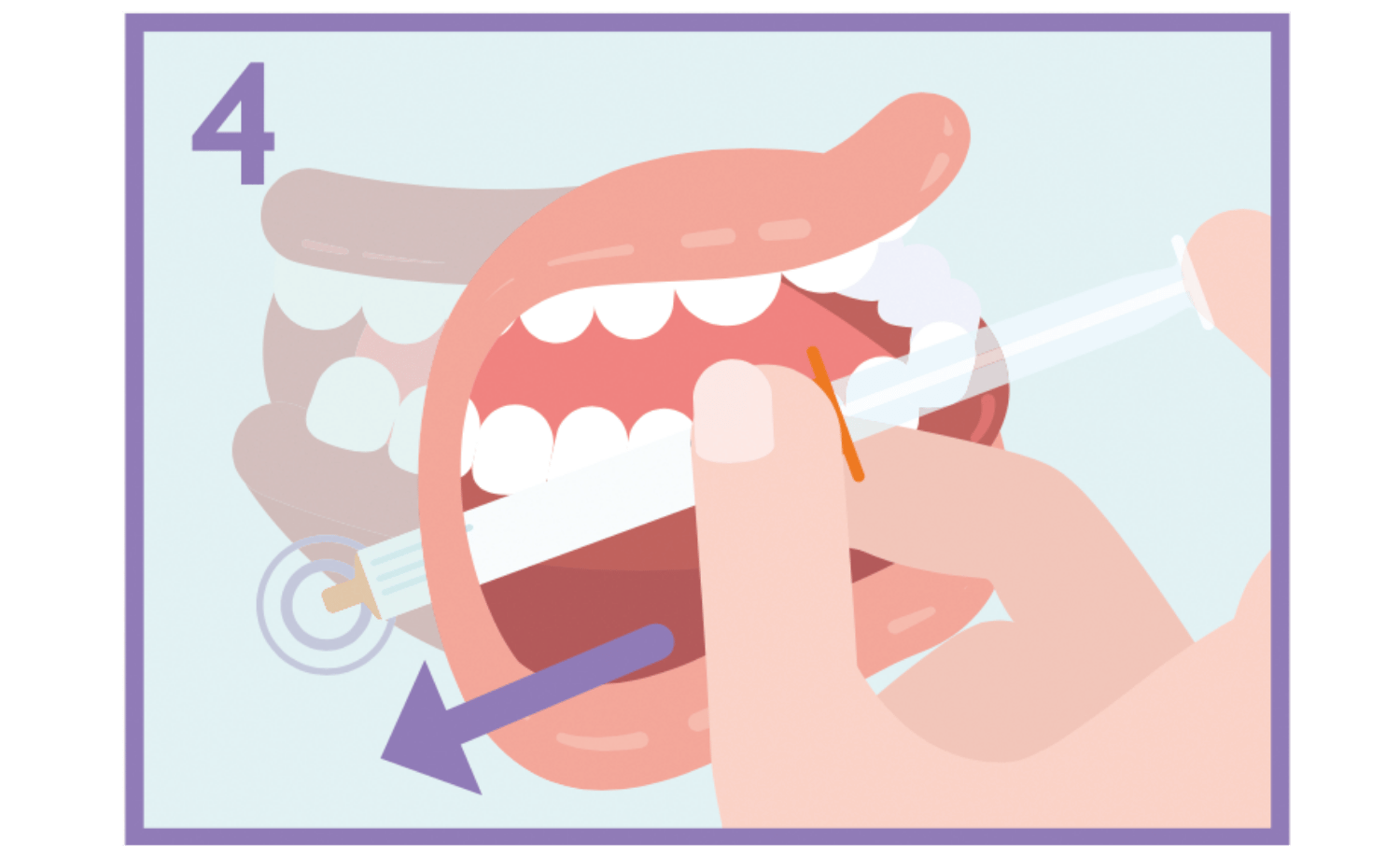
Put the syringe into the back of the space between the inside of the cheek and the lower gum.
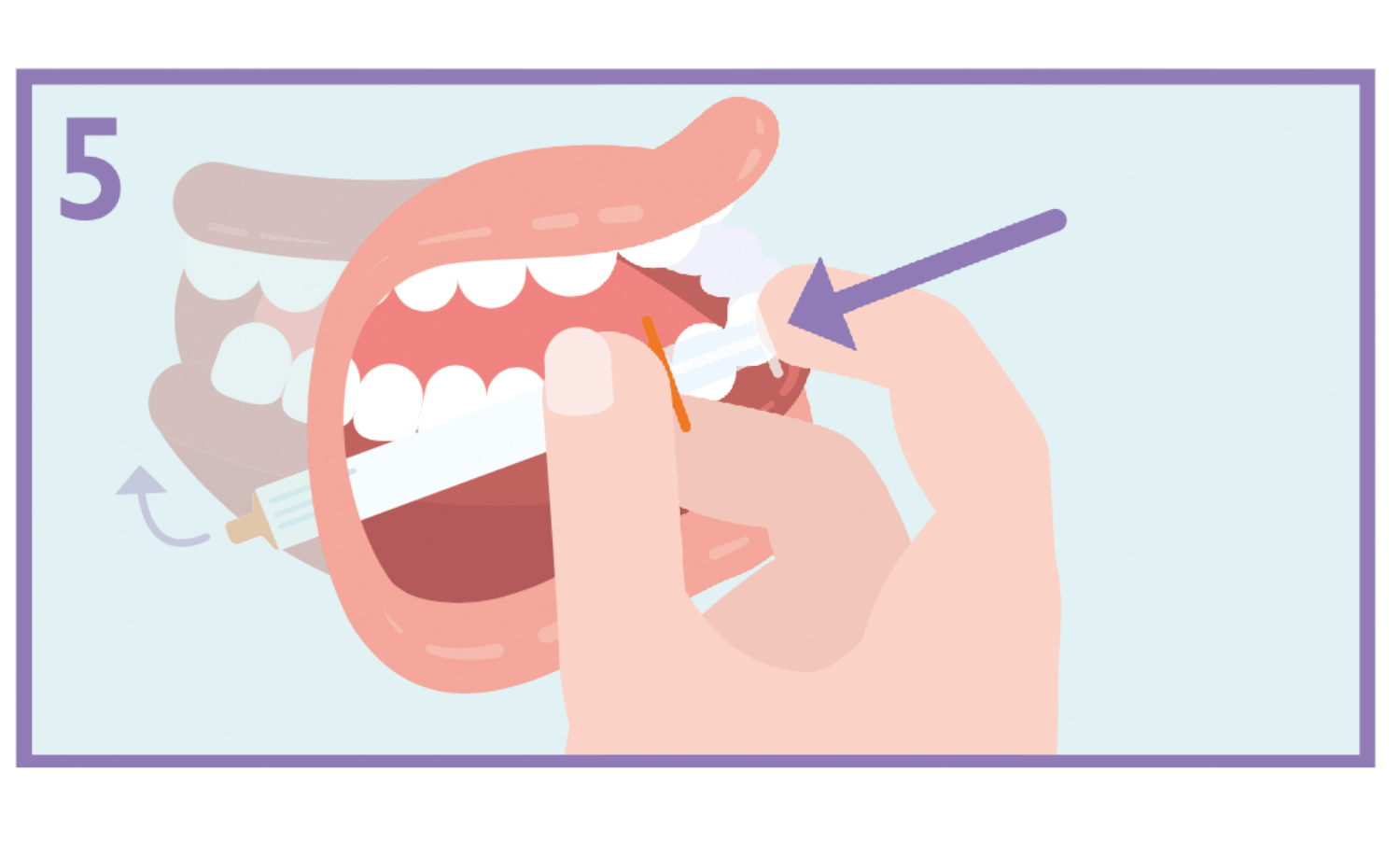
Slowly press the syringe plunger until the plunger stops. The full amount of solution should be inserted slowly into the space between the gum and the cheek (buccal cavity). If prescribed by your doctor (for larger volumes and/or smaller patients), you can give approximately half the dose slowly into one side of the mouth, then into the other side of the patient’s mouth.

Stay with the patient until the seizure is over. Note the time BUCCOLAM® was given and how long the seizure lasted. Stay with the patient until they are fully recovered.
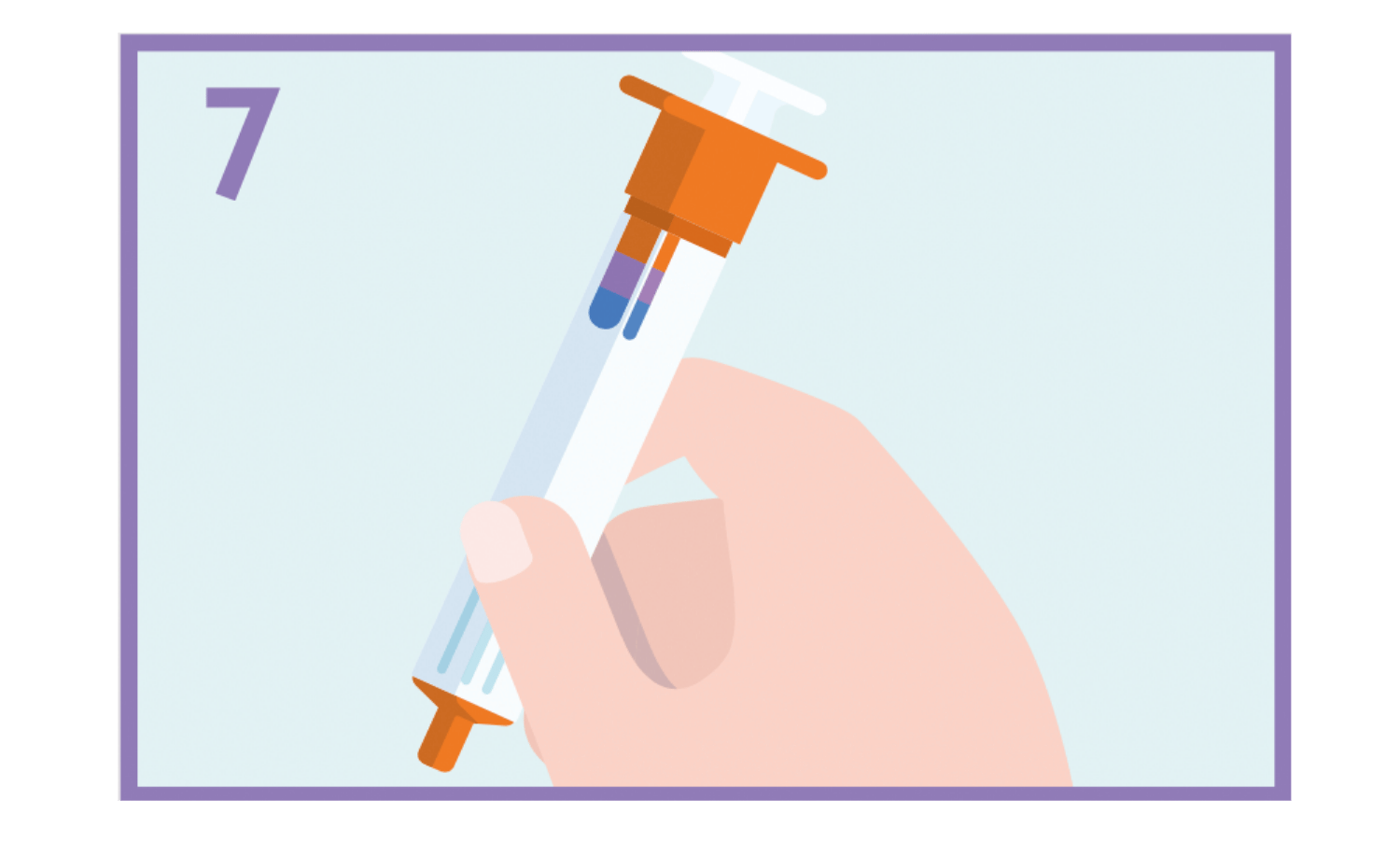
Keep the empty syringe to give it to the doctor or paramedic if they have been called.
If 10 minutes after administering
a single dose of midazolam
the seizure has not stopped:
Parents and carers should only
administer a single dose of
BUCCOLAM®. Additional doses
should not be administered.
If seizures re-occur after an initial
response, additional doses should
not be administered without prior
medical advice.
The time when BUCCOLAM® was
administered must be annotated.
Patients should be accompanied, and should not drive a vehicle, ride a bike or operate machinery,
until they are fully recovered.
Advise the patient, family member and/ or carer of the following:
When to call an ambulance
ALWAYS follow the treatment advice provided by a healthcare professional. If in any doubt, call for immediate medical help if:
If the patient vomits
References
1. Buccolam 2.5 mg , 5 mg, 7.5 mg and 10 mg Summaries of Product Characteristics
2. Buccolam 2.5 mg , 5 mg, 7.5 mg and 10 mg Patient Information Leaflet
This information is intended for use by healthcare professionals.
NXUK/E/1125/01 December 2025
The content of this website is for the exclusive use of healthcare professionals authorised to prescribe, dispense, indicate, use or authorise the dispensing of prescription medicines in United Kingdom.
Click Accept if you are a healthcare professional in United Kingdom and wish to continue on this site or Exit to be redirected to the Neuraxpharm United Kingdom website.