
This is a promotional website with content intended for UK healthcare professionals only
Adverse events should be reported. Healthcare professionals are asked to report any suspected adverse reactions. UK reporting forms and information can be found via the Yellow Card Scheme www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Stores. Adverse events should also be reported to Neuraxpharm UK Ltd by emailing pv-uk@neuraxpharm.com
Efficacy in children
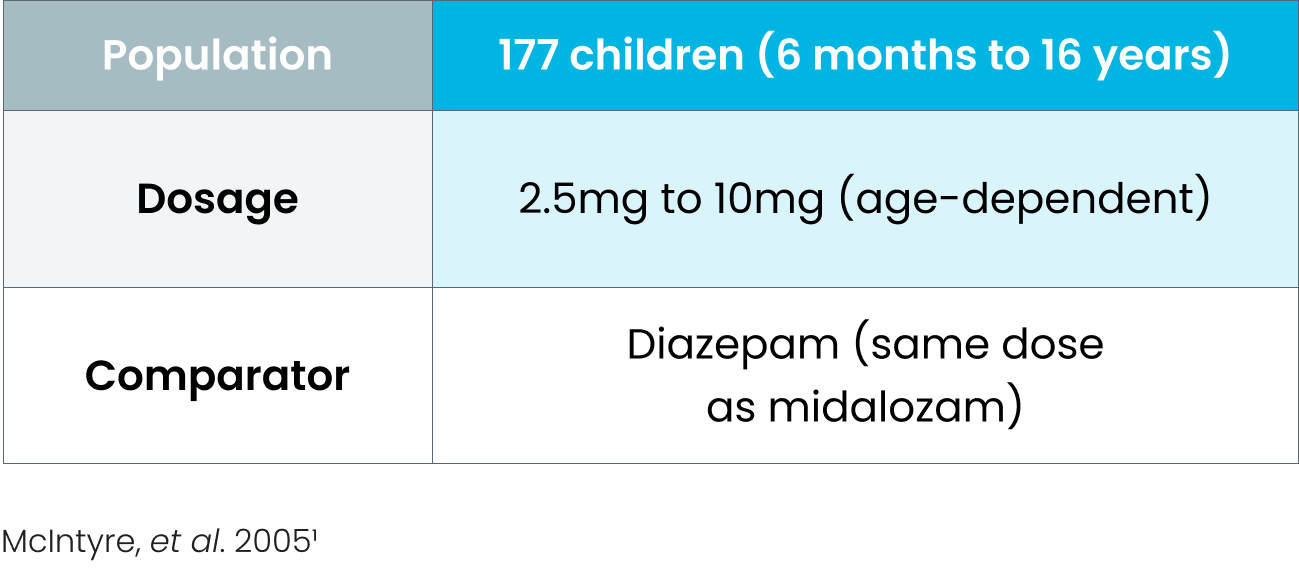
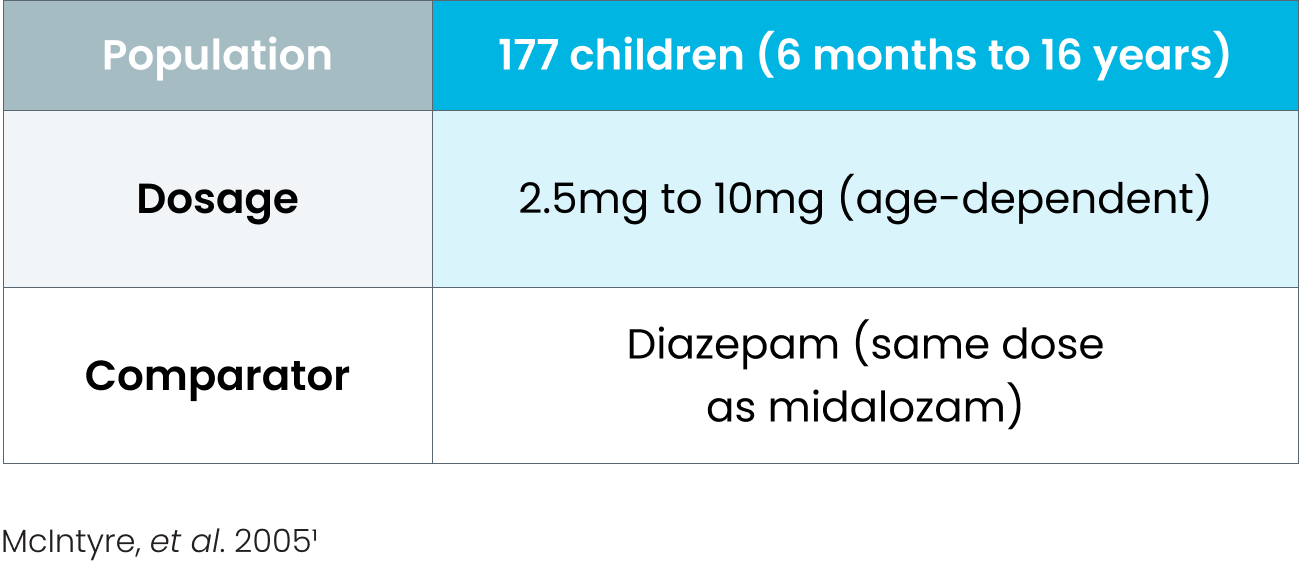
Efficacy of BUCCOLAM oromucosal in managing PACS was established in several controlled studies, comparing it with rectal and intravenous diazepam 1-4
Oromucosal mizadolam presents convenient administration.1-4 These studies, all conducted in hospital setting,1-4 suggest that use of oromucosal midazolam has some advantages compared to rectal diazepam. 1-3
Overview
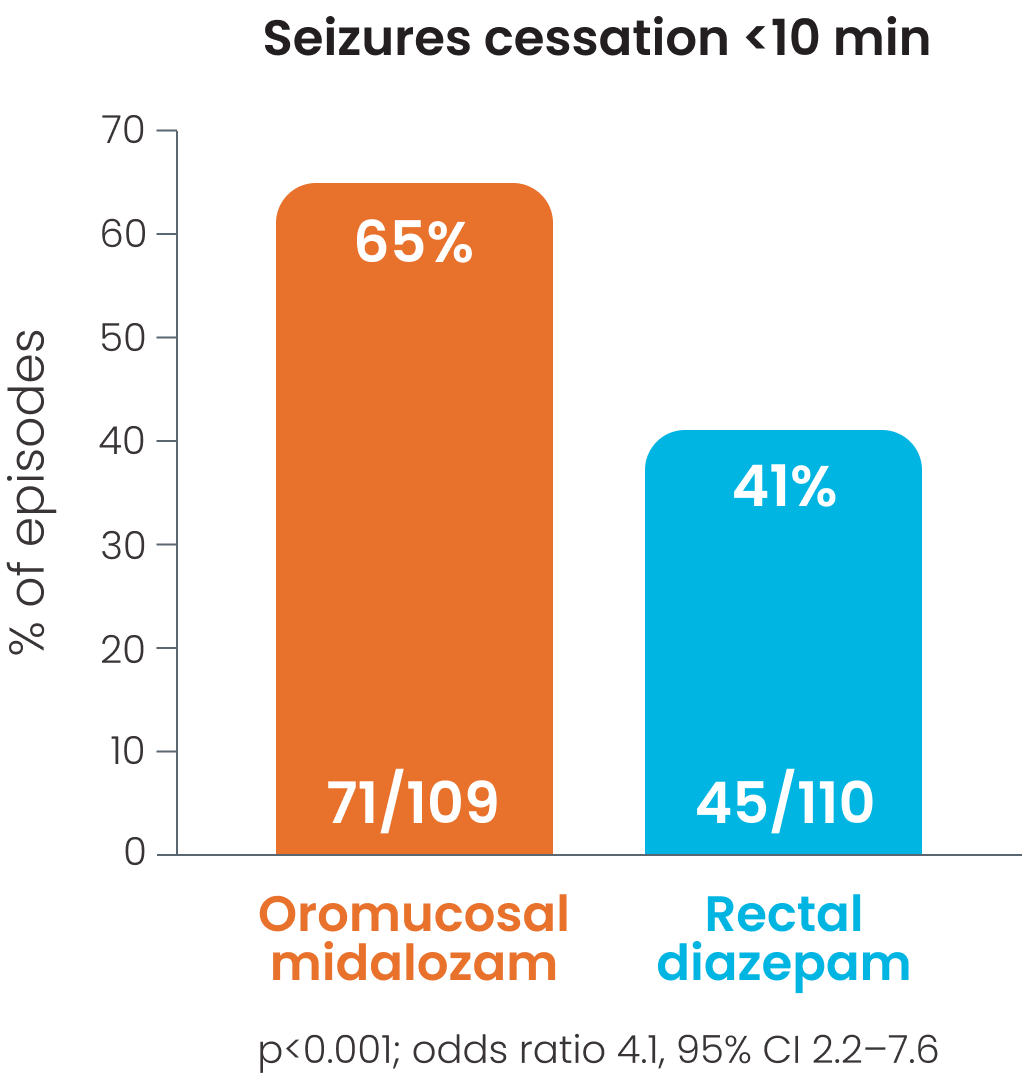
Cessation of visible signs of seizures within 10 minutes was observed in 65% patient episodes in those receiving oromucosal midazolam; p<0.001 vs. rectal diazepam.1
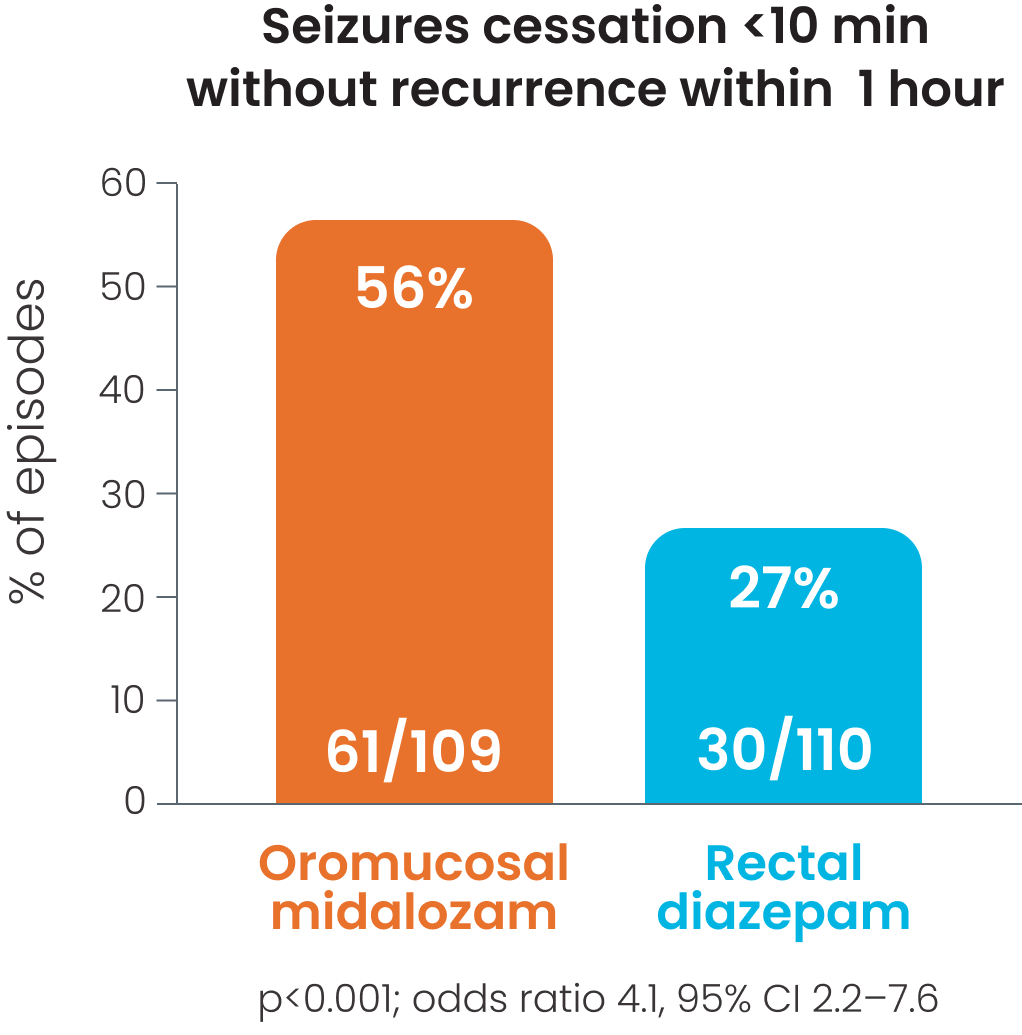
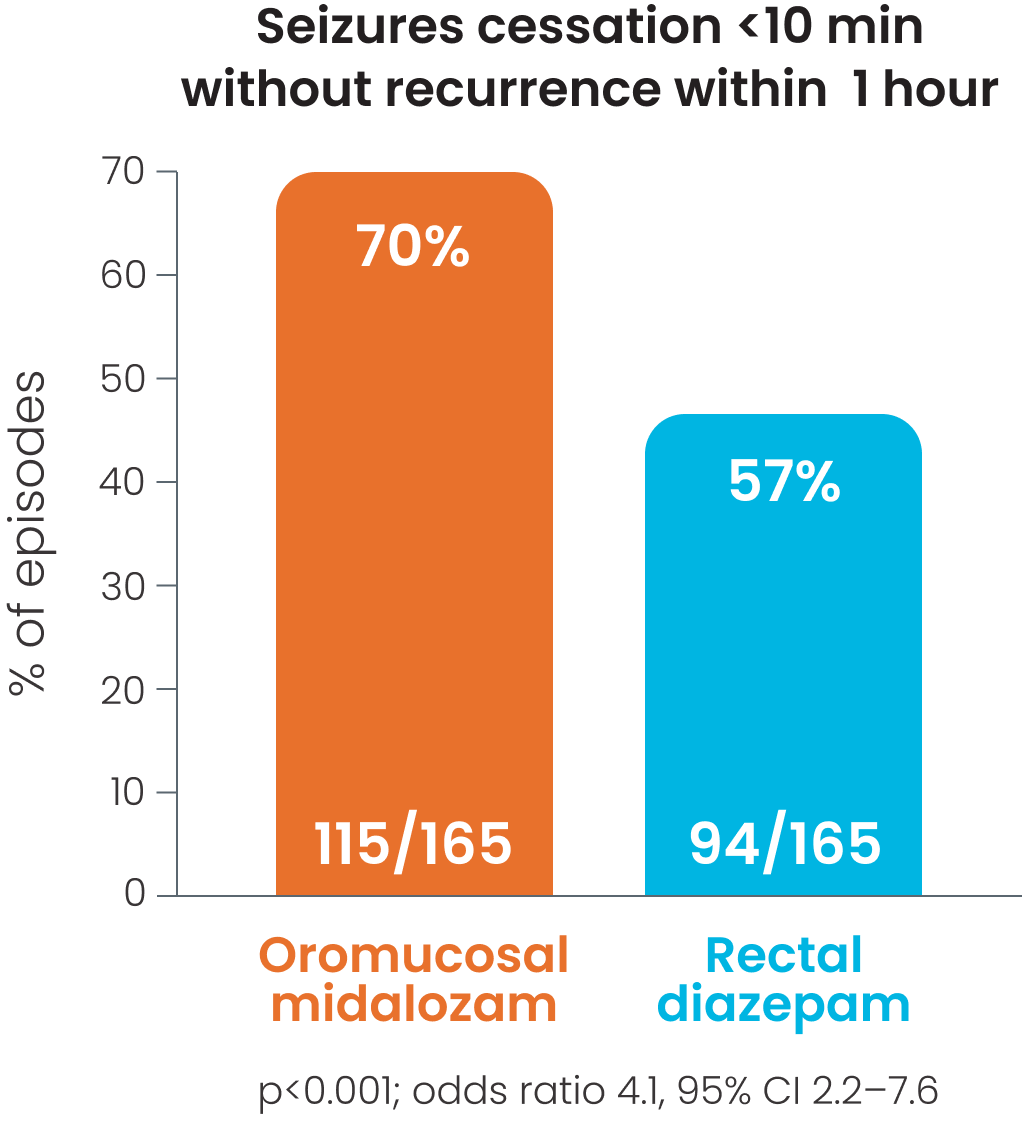
Cessation of visible signs of seizures within 10 minutes without recurrence within 1 hour after was observed in 56%1 -70%2 of patients receiving oromucosal midazolam; p<0.0011, p=0.026.2
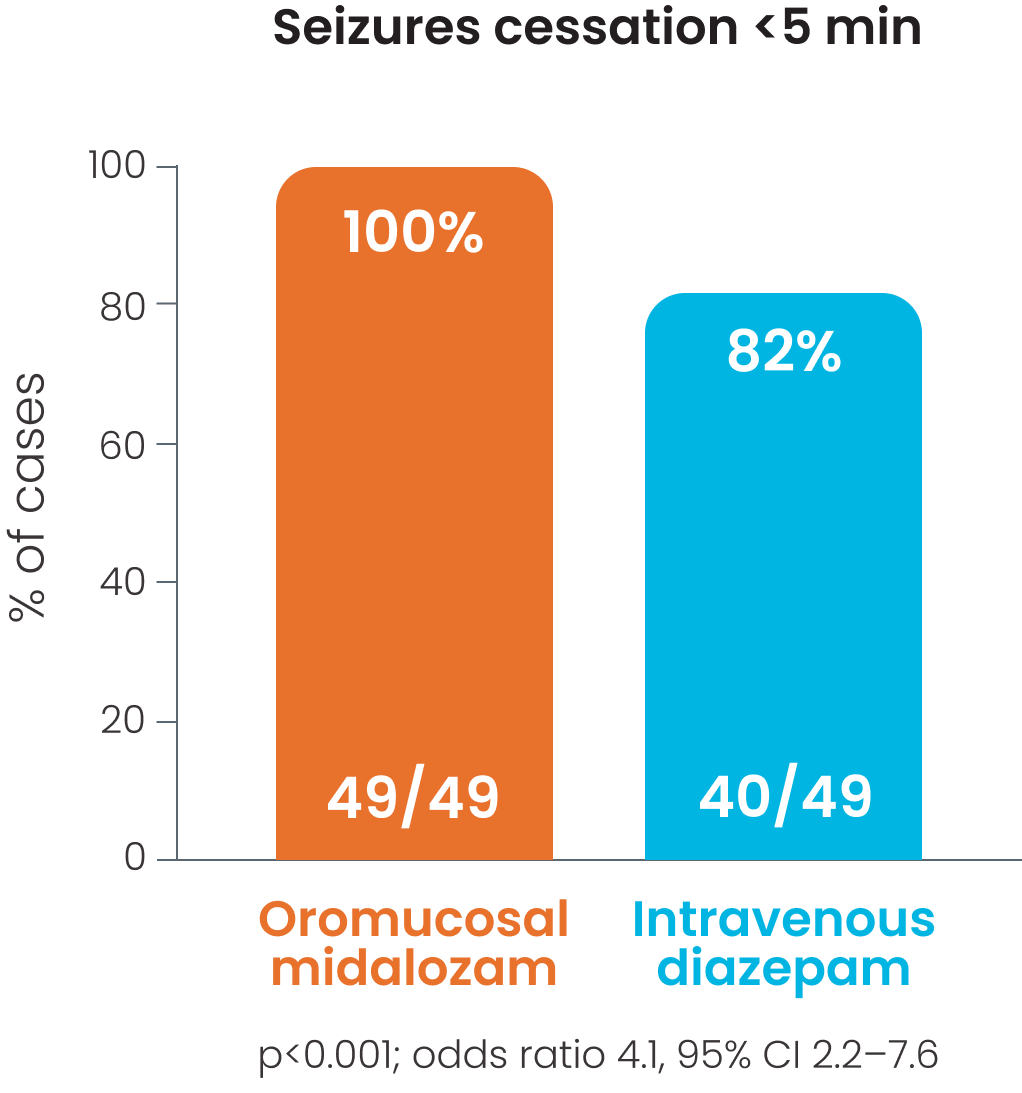
Cessation of all motor activity within 5 minutes was observed in 85-100% of patients receiving oromucosal midazolam; p<0.001.3
94% (46/49) of parents expressed
satisfaction with their childs' treatment and route of drug administration midazolam
administration, compared
to 14% satisfaction in the
rectal diazepam group;
p<0.001.3
In this study administration was made by trained personnel
Study details
Cessation of visible signs of seizures within 10 minutes was observed in 65% patient episodes in those receiving oromucosal midazolam.1
Cessation of visible signs of seizures within 10 minutes without recurrence within 1 hour was observed in 56% of patients receiving oromucosal midazolam.1,2
Cessation of visible signs of seizures within 10 minutes without recurrence within 1 hour was observed in 70% of patients receiving oromucosal midazolam.2
Cessation of all motor activity within 5 minutes was observed in 85-100% of patients receiving oromucosal midazolam.3
The main outcome variable was cessation of all motor activity. This to be achieved in less than 5 min without respiratory depression and without another seizure within 1 hour, otherwise treatment considered a failure.
BUCCOLAM® use in adults
The 10 mg dose was identified as yielding exposures in adults consistent with those of demonstrated efficacy in children, in this pharmacokinetic modelling study.6 This allows the extrapolation that similar therapeutic outcomes can be achieved in adults as in children.6
Additionally, a number of other clinical studies5, 7, 8 using buccally administered midazolam in adults have demonstrated the effectiveness of its use as rescue medication when administered in either hospital or community settings.
The standard dose recommended for patients aged 10 years and above, including adults is 10 mg pre-filled syringe5
Tolerability
BUCCOLAM® safety profile5
The table summarises the adverse reactions* that have been reported in association with the use of oromucosal midazolam.9
Click here to access BUCCOLAM (midazolam) Summaries of Product Characteristics
Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness:
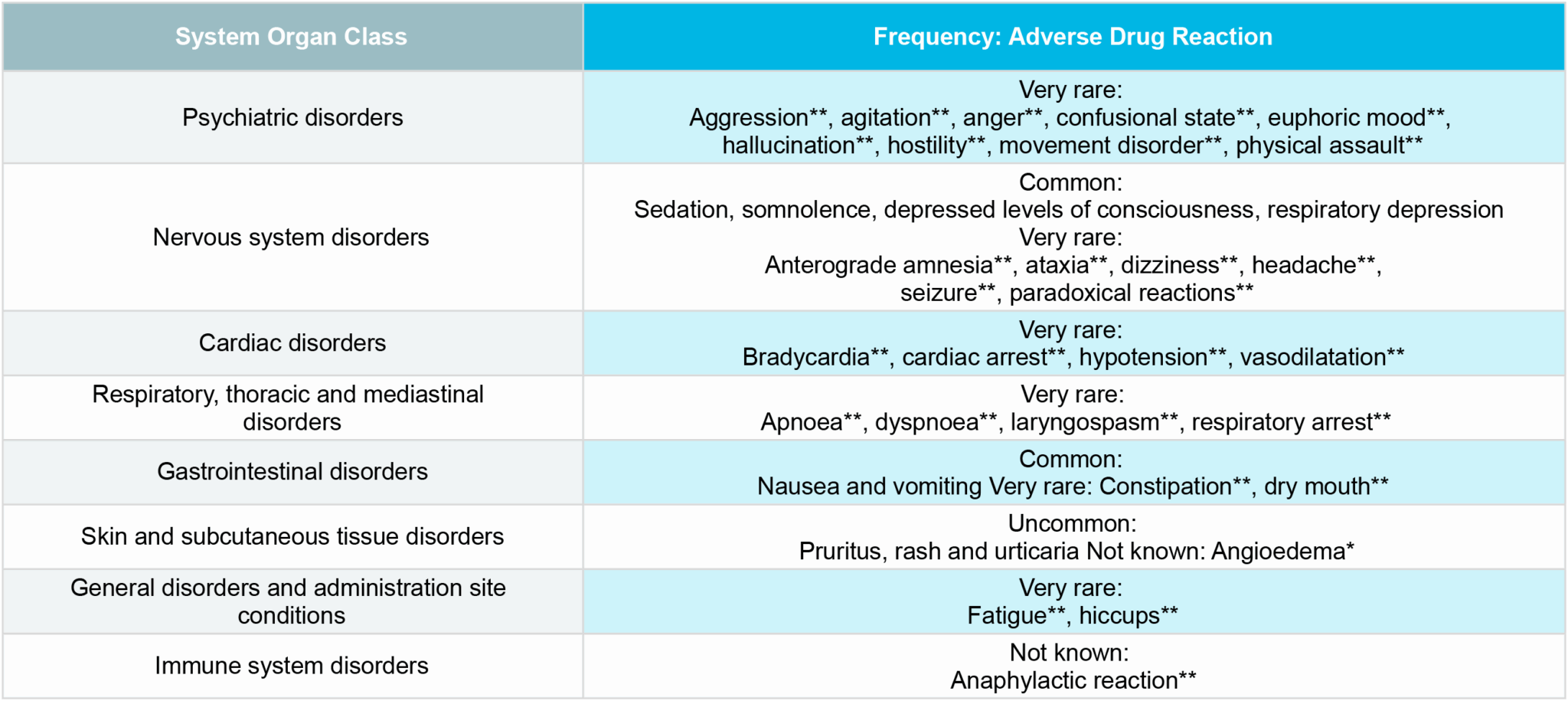
**These adverse reactions have been reported to occur when midazolam is injected in children and/or adults, which may be of relevance to oromucosal administration.
* ADR identified from post-marketing experience.
Serious undesirable effects can include angioedema and anaphylactic reaction 5
Published clinical studies show that oromucosal midazolam was administered to approximately 443 children and 224 adults with seizures.5
• The rates of respiratory depression were up to 5% (this is a known complication of convulsive seizures as well as being related to midazolam use).5
• One episode of pruritus was possibly attributed to the use of buccal midazolam.2, 5
• The frequency and severity of adverse drug reactions, in paediatric studies, were similar to those reported in the comparative group using rectal diazepam.5
Life-threatening incidents are more likely to occur in those with pre-existing respiratory insufficiency or impaired cardiac function, particularly when a high dosage is administered.5
• Contraindications5
• Warnings and precautions for use5
• Fertility, pregnancy and lactation5
Midazolam should be used with caution in patients with chronic respiratory insufficiency because midazolam may further depress respiration
Paediatric patients aged 3 to 6 months
Midazolam should be used with caution in elderly patients and patients with chronic renal failure, impaired hepatic or cardiac function. Midazolam may accumulate in patients with chronic renal failure or impaired hepatic function whilst in patients with impaired cardiac function it may cause decreased clearance of midazolam
Debilitated patients are more prone to the central nervous system (CNS) effects of benzodiazepines and, therefore, lower doses may be required
Midazolam should be avoided in patients with a medical history of alcohol or drug abuse
Midazolam may cause anterograde amnesia
* For more detailed information please see the SmPC.9
• Interactions5
• Effects on ability to drive and use machines5
Sedation, amnesia, impaired attention and impaired muscular function may adversely affect the ability to drive, ride a bicycle or use machines. After receiving midazolam, the patient should be warned not to drive a vehicle or operate a machine until completely recovered.
Midazolam overdose can present a threat to life if the patient has pre-existing respiratory or cardiac insufficiency, or when combined with other CNS depressants (including alcohol).
Overdose of benzodiazepines is usually manifested by degrees of central nervous system depression ranging from drowsiness to coma to death.
Mild cases
Serious cases
Overdose management5
In the management of overdose with any medicinal product, it should be borne in mind that multiple agents may have been taken. Special attention should be paid to respiratory and cardiovascular functions in intensive care.
Conscious patient
Induce vomiting
(within 1 hour)
Unconscious patient
Gastric lavage
(with airway protection)
Activated charcoal
Others
References
1 McIntyre J, Robertson S, Norris E, Appleton R, Whitehouse WP, Phillips B, Martland T, Berry K, Collier J, Smith S, Choonara I. Safety and efficacy of buccal midazolam versus rectal diazepam for emergency treatment of seizures in children: a randomised controlled trial. Lancet. 2005 Jul 16-22;366(9481):205-10. doi: 10.1016/S0140-6736(05)66909-7. PMID: 16023510.
2 Mpimbaza A, Ndeezi G, Staedke S, Rosenthal PJ, Byarugaba J. Comparison of buccal midazolam with rectal diazepam in the treatment of prolonged seizures in Ugandan children: a randomized clinical trial. Pediatrics. 2008 Jan;121(1):e58-64. doi: 10.1542/peds.2007-0930. PMID: 18166545.
3 Ashrafi MR, Khosroshahi N, Karimi P, Malamiri RA, Bavarian B, Zarch AV, Mirzaei M, Kompani F. Efficacy and usability of buccal midazolam in controlling acute prolonged convulsive seizures in children. Eur J Paediatr Neurol. 2010 Sep;14(5):434-8. doi: 10.1016/j.ejpn.2010.05.009. Epub 2010 Jun 15. PMID: 20554464.
4 Talukdar B, Chakrabarty B. Efficacy of buccal midazolam compared to intravenous diazepam in controlling convulsions in children: a randomized controlled trial. Brain Dev. 2009 Nov;31(10):744-9. doi: 10.1016/j.braindev.2008.11.006. Epub 2008 Dec 27. PMID: 19114297.
5. Buccolam 2.5 mg , 5 mg, 7.5 mg and 10 mg Summaries of Product Characteristics
6 Lopez Bermudo C, Carreño, M, Valiante C et al. Definition of adult posology of midazolam oromucosal solution for prolongedseizures based on a population pharmacokinetic model. Poster presented EEC 2024. 15th European Epilepsy Congress 2024 Sep 7-11; Rome, Italy
7 Nakken, K.O. and Lossius, M.I. (2011), Buccal midazolam or rectal diazepam for treatment of residential adult patients with serial seizures or status epilepticus. Acta Neurologica Scandinavica, 124: 99-103. https://doi.org/10.1111/j.1600-0404.2010.01474.x
8 Shankar R, Goodwin M, Toland J, Boyle A, Grant A, Pearson J, Storer A, Higgins R, Hudson S, Reuber M. Oro-mucosal midazolam maleate: Use and effectiveness in adults with epilepsy in the UK. Epilepsy Behav. 2021 Oct;123:108242. doi: 10.1016/j.yebeh.2021.108242. Epub 2021 Aug 7. PMID: 34371288.
This information is intended for use by healthcare professionals.
NXUK/E/1125/01 December 2025
The content of this website is for the exclusive use of healthcare professionals authorised to prescribe, dispense, indicate, use or authorise the dispensing of prescription medicines in United Kingdom.
Click Accept if you are a healthcare professional in United Kingdom and wish to continue on this site or Exit to be redirected to the Neuraxpharm United Kingdom website.